On FA Awareness Day, Friedreich’s Ataxia Research Advocate Is Upbeat

Ron Bartek, founder of the Friedreich's Ataxia Research Alliance, at WODC2019. (Photo by Isaura de los Santos)
“Excited and beyond belief” is how Ron Bartek, co-founder and president of the Friedreich’s Ataxia Research Alliance (FARA), describes current prospects for finding treatments for the incurable genetic disease that claimed his son, Keith, at age 24.
Bartek spoke to Friedreich’s Ataxia News on the sidelines of the recent 2019 World Orphan Drug Congress USA, in Oxon Hill, Maryland. Some of the 30 to 35 companies working on potential ataxia treatments attended the event, as did a number of nonprofit groups such as FARA that advocate for rare-disease patients and their families.
“At this conference, the majority of presentations centered on the tremendous importance of patient advocacy groups working with our industry and government partners,” Bartek said. “We knew from the beginning there was absolutely no way we would accomplish anything by ourselves. So we started working with anybody and everybody we could. Eventually that led to industry partners coming to our side.”
Friedreich’s Ataxia Awareness Day — celebrated the third Saturday in May — is May 18 this year. FARA aims to raise $250,000 through the worldwide “Lend Us Some Muscle” campaign, which was established two years ago in Australia to help find a cure for FA.
“We are just excited beyond belief about this year,” Bartek said. “This is the year regenerative medicines are coming to our doorstep. That includes enzyme replacement therapies and gene therapy. We’re expecting that this year, we’ll have at least two, if not three, clinical trials commencing in those kinds of regenerative medicines.”
What thrills Bartek the most, he said, is that in 2019, “we will actually begin clinical trials in regenerative medicines — or approaches that have the prospect of addressing the underlying cause of FA, which is low levels of frataxin protein.”
The FA treatment pipeline
In addition, several companies and academic research institutes are working on gene therapies to cure FA, including Pfizer, Voyager Therapeutics/Neurocrine and PTC Therapeutics, as well as the University of Florida’s Powell Gene Therapy Center, the University of Pennsylvania Gene Therapy Program, and Weill Cornell Medicine in New York.
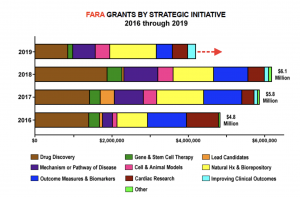
In 2018, FARA funded $6.1 million in research, up 5.2% from the $5.8 million in grants awarded in 2017. Last year, the organization funded 15 new grants in the these strategic initiatives: drug discovery (4); gene and stem cell therapy (1); mechanisms or pathway of disease (3); cell and animal models (3); and outcome measures and biomarkers (4).
The organization also renewed funding for 23 projects spanning nearly all of these strategic areas.
More than a dozen specific therapies are in the FA treatment pipeline, at various stages ranging from discovery and preclinical development to Phase III trials. Furthest along are Omaveloxolone (RTA-408) from Reata Pharmaceuticals in collaboration with AbbVie, which aims to improve mitochondrial function and reduce oxidative stress, and TAK-831 from Takeda Pharmaceuticals, aimed at relieving some FA symptoms.
Other potential therapies seek to add, stabilize or enhance frataxin — the protein largely missing in FA patients — or increase FA gene expression (protein production).
“We have no companies already in clinical trials for gene therapies or protein replacement, but several are on the threshold,” he said. “We’re probably going to need them all, because no single therapy can be one-and-done. Not all of our patients will respond to a single therapy or a curative. It’s going to be a cocktail approach.”
Up to 20,000 FA patients worldwide
For most of his professional life, Bartek’s world was more about nuclear deterrence and NATO than proteins and pluripotent stem cells.
“Before 1997, I wouldn’t have been able to spell Friedreich’s ataxia,” he said. But then, 11-year-old Keith was diagnosed with FA.
“We mad-scrambled onto our computers and tried to figure out what this disease was. We realized he would lose his vision and speech, and probably be in a wheelchair by 15 with congestive heart failure. All that happened to our son; he got all those symptoms.”
Bartek, who has a graduate degree in Russian studies, worked in military and political affairs on Capitol Hill. His wife worked for former U.S. Rep. Billy Tauzin, a Republican lawmaker from southern Louisiana who later went on to become president of the Pharmaceutical Research and Manufacturers of America.
According to Bartek, about 1 in 90 Americans in general are carriers of FA, and that about 5,000 Americans have the disease, with perhaps 15,000 to 20,000 FA patients worldwide.
FA almost never strikes black people or those of Far East ancestry such as Chinese or Japanese. However, in addition to North, Central and South America, Australia and New Zealand, the disease occurs in South Asian countries such as India and Pakistan, and throughout the Middle East.
World’s largest FA registry
In its first year, the Pennsylvania-based charity raised $100,000 — half of which came from the U.S. National Institutes of Health — to support the world’s first scientific conference on FA, attended by 80 researchers.
“We started FARA in 1998, a year after our son was diagnosed, with a board of directors of 11 people, three of which were scientists. The rest were parents and one adult patient,” he said.
The following year, FARA’s annual budget doubled, he said. By 2006, Bartek had quit his job as president of a consulting firm that advised clients seeking business opportunities in energy and national security, including working with former Soviet republics.
FARA’s budget now approaches $8 million, about 93% of which goes to scientific research. The organization has nine full-time employees.
“Our assets are the education and motivation of our patient community, the patient registry, the natural history data and translational tools,” he said.
At the moment, 3,669 patients are in the FARA registry — making it the largest of its kind in the world — including 1,749 from the United States and 510 from Brazil. The organization also has 1,300 FA patients in its natural history database, though the two structures are kept separate.
“We actually help companies design clinical trials and pick the right patients who are best suited to their therapeutic approach,” Bartek said. “We recruit those clinical trials in a heartbeat. That’s remarkably important because so many trials fail due to lack of recruitment.”