Voyager Therapeutics to Host CNS Disease R&D Day and Webcast April 29
Written by |

 Cambridge, Massachusetts-based clinical-stage gene therapy company Voyager Therapeutics will host an R&D Day from 8:30 to 11 a.m. Eastern Time on Friday, April 29, in New York City. Voyager specializes in the development of treatments for fatal and debilitating diseases of the central nervous system (CNS) such as Friedreich’s ataxia, ALS, Parkinson’s disease, Huntington’s disease, spinal muscular atrophy (SMA), and others.
Cambridge, Massachusetts-based clinical-stage gene therapy company Voyager Therapeutics will host an R&D Day from 8:30 to 11 a.m. Eastern Time on Friday, April 29, in New York City. Voyager specializes in the development of treatments for fatal and debilitating diseases of the central nervous system (CNS) such as Friedreich’s ataxia, ALS, Parkinson’s disease, Huntington’s disease, spinal muscular atrophy (SMA), and others.
The R&D day presentations by members of Voyager’s management team and guest speakers include:
Unmet Medical Needs in Parkinson’s Disease and Potential Treatment Using Gene Therapy
Andrew S. Feigin, M.D., director, Experimental Therapeutics for Movement Disorders, Feinstein Institute; director, Huntington’s Disease Society of America Center of Excellence, North Shore University Hospital.
Overview of VY-AADC01 for Parkinson’s Disease
Bernard Ravina, M.D., vice president of clinical development, Voyager Therapeutics.
Role of Superoxide Dismutase 1 in ALS and the Potential for Targeted Treatment Using Gene Therapy
Robert H. Brown, D.Phil., M.D., professor and chair of neurology, University of Massachusetts Medical School; director, Day Neuromuscular Research Laboratory, University of Massachusetts Medical School.
Overview of VY-SOD101 for a Monogenic Form of ALS
Dinah Sah, Ph.D., senior vice president, Neuroscience, Voyager Therapeutics.
New Opportunities in CNS Gene Therapy
Steve Paul, M.D., president and CEO, Voyager Therapeutics.
A live audio webcast of the R&D Day and replay will be available online from the Investors & Media section of Voyager’s website at https://www.voyagertherapeutics.com. A replay of the presentation will be posted on the Voyager website about one hour after the live event and will be available for 30 days following the presentation.
Voyager Therapeutics is committed to advancing the field of AAV (adeno-associated virus) gene therapy through innovation and investment in vector engineering and optimization, manufacturing and dosing and delivery techniques, with development programs in progress for new treatments to address several CNS diseases, including Friedreich’s ataxia.
The company says that for each of these disorders, there exists dire need for new therapies. Depending on the disease, Voyager’s gene therapies will use either gene replacement or gene knockdown techniques. By significantly increasing or decreasing production of relevant proteins at targeted sites within the central nervous system, the goal is to address the underlying biology of the disease and make a meaningful difference for patients.
For example, the company notes that Friedreich’s ataxia is the most common hereditary ataxia, with approximately 8,000 patients living with the disease in the United States and Europe. Friedreich’s ataxia patients have a genetic mutation in the FXN gene, which limits production of the protein frataxin. This causes a range of debilitating symptoms and complications, including muscle weakness, impaired vision, hearing, and speech, as well as aggressive scoliosis, diabetes, heart disease, and difficulty breathing.
By delivering a functional version of the FXN gene to targeted cells in the CNS, Voyager’s goal is to increase levels of frataxin and have a meaningful impact on the progression of the disease.
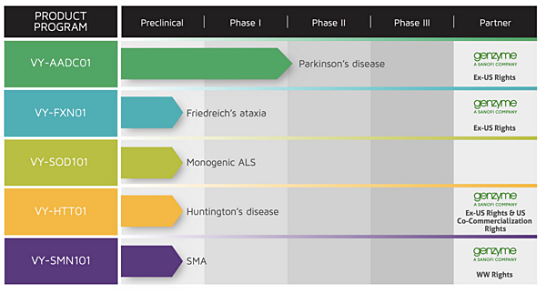
Voyager’s development pipeline includes VY-AADC01 for Parkinson’s disease, which is in an ongoing Phase 1b study, as well as preclinical programs VY-FXN01 for Friedreich’s ataxia, VY-SOD101 for a monogenic form of amyotrophic lateral sclerosis (ALS), VY-HTT01 for Huntington’s disease, and VY-SMN101 for spinal muscular atrophy (SMA).
Voyager, which was founded by scientific and clinical leaders in the fields of AAV gene therapy, expressed RNA interference and neuroscience, has broad strategic collaborations with Sanofi Genzyme, the specialty care global business unit of Sanofi, and the University of Massachusetts Medical School.
For more information, visit
https://www.voyagertherapeutics.com.