Reactivating Nrf2 Pathway Restores Nerve Cell Development in FA, Mouse Study Suggests
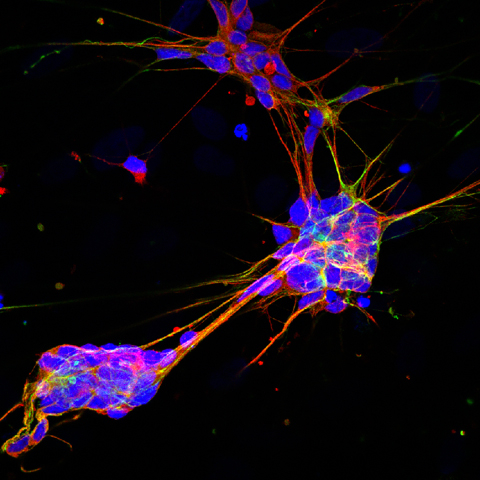
Reactivativation of the Nrf2 pathway, which is impaired in the nerve cells — or neurons — of patients with Friedreich’s ataxia (FA), leads to the re-establishment of proper neuron development and maturation, a mouse study shows.
The study, “Nrf2 Induction Re-establishes a Proper Neuronal Differentiation Program in Friedreich’s Ataxia Neural Stem Cells,” was published in the journal Frontier in Cellular Neuroscience.
FA is a genetic disease in which patients have low levels of the protein frataxin.
While there may be no obvious signs of the disease in the first five to 10 years of life, FA patients develop cognitive differences and defects in brain structures earlier than what can be seen through neuroimaging techniques.
Several studies suggest problems due to frataxin deficiency arise during fetal development. However, there is a lack of research on how low levels of frataxin affect neurogenesis, or the development of neurons in a fetus.
Recent studies have shown that a mouse model of FA, the frataxin knockin/knockout (KIKO) mouse, exhibits defects that closely resemble human clinical symptoms.
Before the onset of FA symptoms, researchers noted that these mice develop abnormalities in the cerebellum, a region of the brain responsible for voluntary movements and speech. Thus, the KIKO mouse model is a useful tool to examine early disease-related changes.
Frataxin deficiency appears to reduce the expression of Nrf2, a type of protein, in human FA cells and in mouse models of the disease.
Nrf2 regulates the expression of antioxidant enzymes, and several studies have shown that induction of the Nrf2 signaling pathway can ease neurological problems.
Nrf2 also plays a significant role in the development of neurons, and according to studies, it helps increase proliferation (cell growth) and self-renewal of neural stem cells (cells that eventually mature into neurons).
In this study, the researchers analyzed the Nrf2 expression in neural stem cells (NSCs) isolated from the embryonic brain of the FA mouse model and evaluated whether an imbalance in the Nrf2 signaling pathway is associated with early defects in neurogenesis.
First, they showed that NSCs isolated from the mice have lower expression of Nrf2 and Nrf2 target genes than NSCs from healthy mice.
Next, they found that NSCs isolated from affected mice had defects in cell proliferation, stemness potential (the ability of cells to self-renew), and differentiation (maturation into fully developed neurons).
Then, the researchers analyzed the ability of two compunds, sulforaphane (SFN) and EPI-743, to induce activation of the Nrf2 pathway. These two investigational therapies “are receiving increasing attention as promising candidates for the treatment of neurodegenerative diseases, including [FA],” the authors wrote.
Results indicated that SFN and EPI-743 both reactivated Nrf2. Additionally, both treatments partially restored proliferation and stemness potential of the NSCs from the FA model.
“We demonstrate that enhancing the expression and activity of the antioxidant response master regulator Nrf2 ameliorates the phenotypic [symptom] defects observed in NSCs, re-establishing a proper differentiation program,” the authors concluded.






