Study of Friedreich’s Ataxia Genetic Network Aims at Making Gene Therapy Possible
Written by |
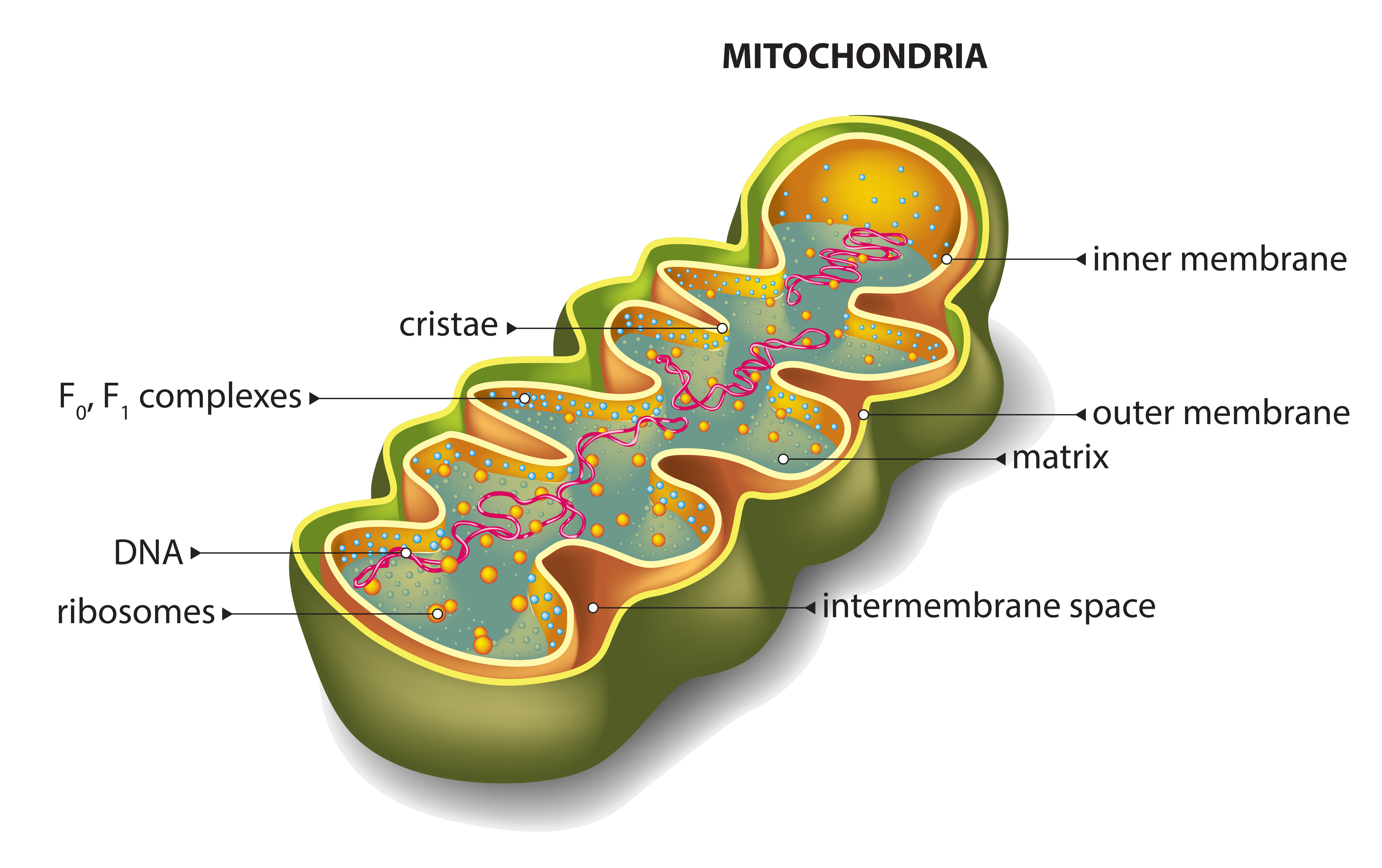
Fibroblasts, or connective tissue cells, from Friedreich’s ataxia patients were used to identify changes in the frataxin (FXN) gene under stress, and to see how it interacts in various regulatory and biological processes. This basic genetics research, in helping to explain and understand the disease, may help to guide the development of gene therapies for Friedreich’s ataxia.
The study, “Characterization of frataxin gene network in Friedreich’s ataxia fibroblasts using the RNA-Seq technique,” was published in the journal Mitochondrion.
Friedreich’s ataxia is known to be associated with a mutation found in the FXN gene that causes a decrease and partial loss of FXN, a mitochondrial protein involved in blood synthesis and homeostasis (maintenance of balance) of cellular iron. Its molecular mechanisms, however, have not been identified.
Because the role of FXN is well-known in oxidative stress and iron metabolism, the researchers proposed a model whereby an FXN deficiency could be used to create oxidative stress and to identify genetic changes occurring from the resulting imbalances. In this model, lack of frataxin would stimulate iron accumulation in mitochondria (a cell’s energy center), lead to oxidative stress, and a create a series of events ending with apoptosis (cell death).
The scientists used RNA sequencing to identify key genes in FXN regulation. They identified 38 genes showing significant differential expression compared to normal controls; of those, 33 showed reduced expression, with the greatest change seen in those related to interferons (in the OAS gene family), predominantly associated with a series of reactions leading to apoptosis.
Results also showed an increase in five of the 38 genes in the FXN regulatory network, one of which functions in mitochondrial processes. Taken in context with the study’s proposed model, the evidence suggests that the FXN protein may influence metabolism and energy production.
Finally, the researchers looked at a possible relationship of the OAS family to a gene in FXN (x123), which is predominantly expressed in muscles. Tests showed that, in the FXN fibroblasts and control samples, the expression of this gene was below detection level; however, the researchers feel further studies will provide insight into the little-known role of the x123 gene.
The key processes found affected by FXN regulatory genes were oxidative stress, interferon-induced apoptosis, DNA damage, and blood clotting. Furthermore, the lymphoblasts and fibroblasts of Friedreich ataxia patients also revealed common cellular pathways, including angiogenesis (formation of new blood vessels), interleukin (proteins involved in immunity) signaling, oxidative stress response, and inflammation.
Overall, the “new insights into frataxin regulation genes and associated cellular pathways will provide a better understanding of the molecular mechanisms underlying this debilitating condition,” the researchers said, possibly paving the way to a gene therapy for the disease.





