Suppressing Two Gene-silencing Enzymes May Hold Promise in FA, Study Shows
Written by |
Simultaneous suppression of two histone methyltransferases (HMTs) significantly increased the activity of the FXN gene in cells derived from people with Friedreich’s ataxia (FA) and in a mouse model of the disease.
However, this boost in FXN activity failed to raise the levels of its resulting protein, frataxin, suggesting the presence of other regulatory mechanisms. Still, further studies using HMT blockers are needed, researchers said.
The study, “HMTase Inhibitors as a Potential Epigenetic-Based Therapeutic Approach for Friedreich’s Ataxia,” was published in the journal Frontiers in Genetics.
FA is caused by excessive repeats of three nucleotides, the building blocks of DNA — one guanine (G) and two adenines (A) — within the FXN gene. This gene contains instructions to produce frataxin, a protein thought to control energy production in mitochondria (the cells’ powerhouses).
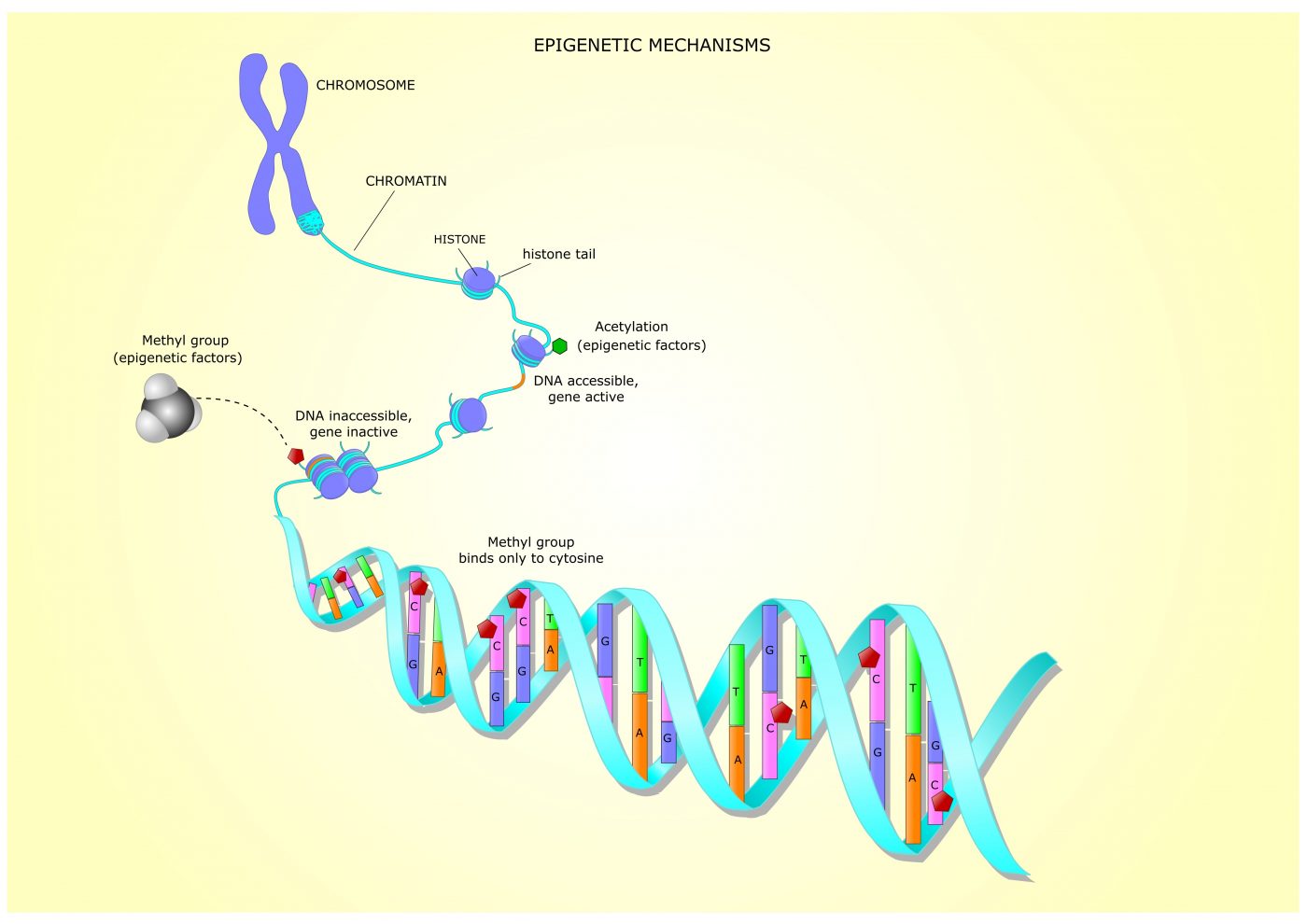
Previous studies have shown that these excessive repeats induce changes that prevent FXN activity. Such changes include epigenetic mechanisms, in which chemical marks are added to DNA or histones, influencing genes’ activities without altering their underlying DNA sequence.
Histones, the proteins that pack DNA and regulate genes’ accessibility and activity, can be modified through the addition or removal of chemical groups by specific enzymes.
Histone modifications near the FXN gene and associated with gene “silencing,” such as those promoted by enzymes called histone deacetylases (HDACs) and HMTs, have been reported in people with FA. While HDACs remove acetyl groups from histones (histone deacetylation), HMTs add methyl groups to histones, which is called histone methylation.
HDAC inhibitors have been evaluated as an experimental approach for the treatment of FA, since some were shown to raise FXN activity and frataxin levels.
However, no studies have explored whether targeting histone methylation holds therapeutic potential.
To address this gap, a team at Brunel University London and their collaborators evaluated the effects of suppressing the activity of two HMTs, known as G9a and EZH2, in cells from FA patients and in a mouse model of the disease.
FXN activity and frataxin protein levels were measured in these lab-grown cells after treatment with BIX0194 (a G9a blocker), GSK126 (an EZH2 inhibitor), or a combination of the two, for three days.
The activity of FXN was assessed through the levels of messenger RNA (mRNA), the molecule derived from DNA and used as template for protein production.
Results showed that cells from FA patients showed significantly higher levels of EZH2 and G9a activity and their repressive histone marks than those from healthy people, supporting the involvement of the corresponding HMTs in FXN “silencing.”
Either treatment led to a significant drop in HMTs activity as well as changes in levels of repressive marks (decreases or increases, depending on the specific marker) in cells from FA patients and healthy people. Notably, no significant effects in genes other than FXN, known as “off targets,” were observed in FA patients’ cells treated with both inhibitors.
In addition, while no changes in FXN activity were observed after treatment with either HMT inhibitor, their combination resulted in a significant increase in the levels of FXN mRNA in cells from both people with FA and the mouse model.
In particular, the combination therapy led to a gradual and significant boost in FXN activity by 15% after two days and by 88% after three days. However, this did not translate into a significant increase in frataxin protein levels at any time point.
“This suggests that there may be other [regulatory] mechanisms at play, affecting either the FXN mRNA stability or frataxin protein [production], stability or degradation that will require further investigation,” the researchers wrote.
Overall, the findings highlight that simultaneous suppression of the two HMTs may have a “beneficial effect, to some extent,” in FA by increasing FXN activity levels, the team said.
They added that although this approach did not result in higher frataxin levels, HMT inhibitors “should still be pursued for further preclinical studies, perhaps with other … epigenetic-based compounds, such as HDAC inhibitors.” Such combinations have shown promising results in fragile X syndrome, another repeat expansion disease, the researchers said.