HDAC Inhibitors Studied as Novel Friedreich’s Ataxia Treatment
 Inhibitors of 2-aminobenzamide histone deacetylase (HDAC) are a proposed new treatment for Friedreich’s ataxia. Applying HDAC inhibitors to neuronal cells derived from Friedreich’s ataxia patients’ induced-pluriopotent stem cells results in an increased expression of frataxin mRNA transcripts and protein. A group of researchers at The Scripps Research Institute in La Jolla California was interested in the mechanism of action of HDACs and conducted the study entitled “Mechanism of Action of 2-Aminobenzamide HDAC Inhibitors in Reversing Gene Silencing in Friedreich’s Ataxia,” published in Frontiers in Neurology.
Inhibitors of 2-aminobenzamide histone deacetylase (HDAC) are a proposed new treatment for Friedreich’s ataxia. Applying HDAC inhibitors to neuronal cells derived from Friedreich’s ataxia patients’ induced-pluriopotent stem cells results in an increased expression of frataxin mRNA transcripts and protein. A group of researchers at The Scripps Research Institute in La Jolla California was interested in the mechanism of action of HDACs and conducted the study entitled “Mechanism of Action of 2-Aminobenzamide HDAC Inhibitors in Reversing Gene Silencing in Friedreich’s Ataxia,” published in Frontiers in Neurology.
“Only compounds targeting class I HDACs 1 and 3 are active in increasing FXN mRNA in [neuronal] cells,” wrote lead author Dr. Elisabetta Soragni from the Department of Cell and Molecular Biology. “Our results shed light on the mechanism whereby HDAC inhibitors increase FXN mRNA levels in Friedreich’s ataxia neuronal cells.”
Non-neuronal tissues are also affected by frataxin deficiency, but the hallmarks of Friedreich’s ataxia are neuronal in nature. Within the nervous system, cells in the dorsal root ganglia and dentate nucleus of the cerebellum are affected by decreased mitochondrial function and decreased enzyme activity.
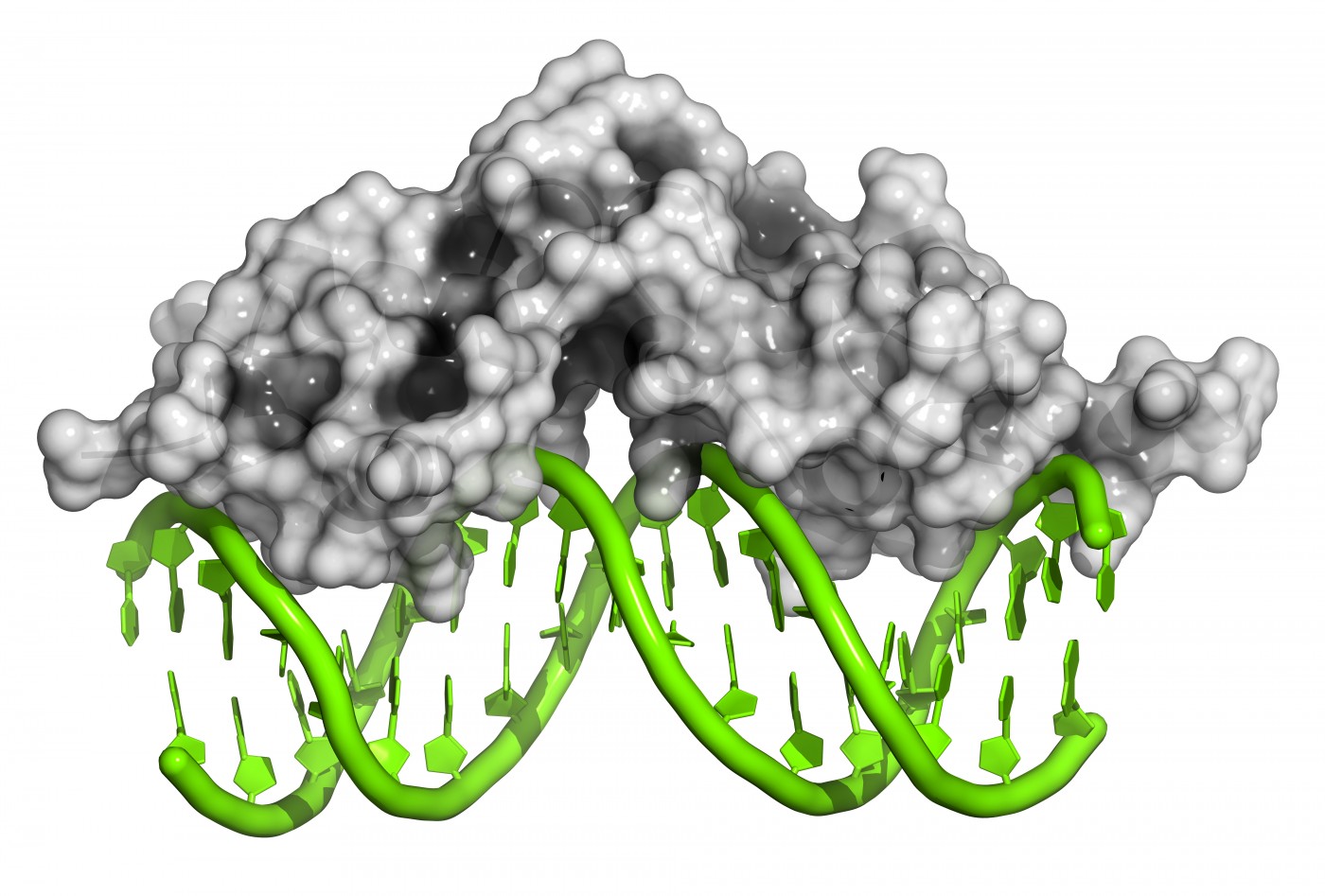
The genetic root cause of Friedreich’s ataxia is a GAA-TTC repeat expansion in the gene encoding frataxin protein. Researchers believe increased DNA methylation, decreased histone acetylation, and increased histone trimethylation contribute to the error in frataxin production, and these are all epigenetic changes. Consequently, in the lab of Dr. Elisabetta Soragni and principal investigator Dr. Joel M. Gottesfeld, a research team screened commercially available HDAC inhibitors in Friedreich’s ataxia lymphoblasts, a type of activated lymphocytes.
The only HDAC inhibitor that yielded a significant increase in frataxin’s mRNA expression was 4b [N1-(2-aminophenyl)-N7-phenylheptanediamide]. This molecule acted on primary lymphocytes to increase gene acetylation, leading to increased frataxin gene expression. 4b was subsequently thoroughly characterized for its actions in Friedreich’s ataxia neurons.
The main take-away message of the study is that the small-molecule HDAC inhibitor seems to act specifically on HDACs 1 and 3 enzymes and has a slow-on/slow-off mechanism of action. It is required for the inhibitor to have a long residence time on its target enzyme.
“Our present and recent findings provide a proof of concept that patient-derived neuronal cells can be a quantitative screening tool for the development of an epigenetic therapy for Friedreich’s ataxia,” wrote Dr. Soragni. However, she did acknowledge that their small-molecule inhibitor in question may not adequately treat Friedreich’s ataxia, as it cannot efficiently penetrate the blood-brain-barrier and is metabolized into potentially dangerous products. Additional chemical alterations may improve the features of this molecule, and future preclinical studies will be used to test its efficacy.